What does the ExoDx prostate cancer test measure?
ExoDx is a simple, non-invasive urine test to assess your risk of having clinically significant or high-grade prostate cancer.
The ExoDx Prostate IntelliScore, or EPI, test provides an individualize risk score help you and your physician make a decision about whether or not to move forward with a prostate biopsy. The ExoDx Prostate Test does not require a digital rectal exam (DRE).
The ExosomeDx test, commonly called the ExoDx test, is used in addition to the PSA test and other standard of care data points (including age, race, and family history) to help healthcare providers determine if an initial biopsy is necessary or if the patient can continue to be monitored. The ExoDx test specifically helps determine if a patient may not have high grade prostate cancer.
ExosomeDx Video
Learn more about the ExosomeDx test, which assesses a patient’s individual risk for high-grade prostate cancer and whether or not a biopsy may be necessary.
Understanding Your ExoDx Results
ExoDx Prostate can be an effective test for using in combination with the PSA and other personal health factors to predict if a patient should be referred for an initial prostate biopsy. See a sample ExoDx results report and learn more about how to interpret the report. Please not that all test results like those of ExoDx should be reviewed with a healthcare provider.
ExoDx FAQs
Below is an initial list of frequently asked questions about the ExosomeDx test.
The PSA test cannot differentiate between prostate cancer and non-cancerous (benign) conditions, such as benign prostatic hyperplasia (BPH). Men who receive a PSA level above 1.5 ng/ML should discuss additional biomarker testing with their healthcare providers prior to considering a prostate biopsy.
Although there are many factors that contribute to your healthcare provider’s decision to conduct a prostate biopsy, there are several urinary and blood based PCMs that may help guide you and your physician through the decision of conducting a prostate biopsy. These include:
Genetic tests also may be worth considering if you have a family history of prostate cancer, breast cancer, ovarian cancer or colorectal cancer. These genetic tests are recommended for men who are determined through genetic counseling to be at potential risk for hereditary cancer:
If you are in the active surveillance stage and wondering about getting more insights into your prostate cancer, these tissue-based tests can be performed on the prostate biopsy you previously had. Results can give you peace of mind or inform your next move.
If you have not had a biopsy and are in the watchful waiting stage based on your initial PSA, DRE, or other tests, consider the PCMs and genetic tests highlighted in Waypoint 1 of the prostate cancer journey.
The intended use population for the ExoDx Prostate Test is:
- Age 50 or over
- PSA of 2-10 ng/mL
- Considering an initial biopsy
There is no evidence to suggest that sexual intercourse would interfere with an ExoDx Prostate result.
No. One of the key benefits of the ExoDx Prostate Test is that it does not require you to have a digital rectal exam (DRE) prior to providing a urine sample for the test. In fact, if you do have a DRE, then you must wait 24 hours before you are able to provide a urine sample for the ExoDx Prostate Test.
The ExoDx Prostate IntelliScore must be ordered by your physician. Your physician will fill out a test requisition, collect a simple urine sample, and send the kit to our laboratory to perform the test. Talk to your doctor or call 1-844-EXOSOME.
The ExoDx test analyzes exosomal RNA from a non-invasive urine sample for three biomarkers known to be expressed in men with high-grade prostate cancer: ERG, PCA3 and SPDEF. You should consult your physician or healthcare provider when reviewing the results of your ExoDx test.
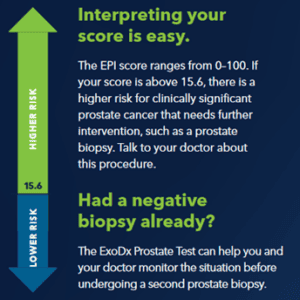
The ExoDx Score is returned as above or below 15.6, which is the test’s cutoff for high grade prostate cancer.
- If below 15.6, there is a decreased likelihood of greater than or equal to Gleason 7 prostate cancer on biopsy.
- If above 15.6, there is an increased likelihood of greater than or equal to Gleason 7 prostate cancer on biopsy.
See a sample ExoDx test result report and learn more about how to interpret these scores.
Learn More
Explore more information about the ExosomeDx test.
- ExoDx Patient website
- ExoDx At-Home Collection Kit
- ExoDx Prostate Test for Risk Assessment of high-grade prostate cancer (HGPCa) Webinar
- A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2–10 ng/ml at Initial Biopsy


